Background: Emicizumab, a monoclonal antibody which functions as a bypassing agent by bridging activated factor IX and factor X in the absence of normal factor VIII levels, has recently been approved for routine prophylaxis against bleeding episodes in both adults and pediatric patients with hemophilia A.
While complete blood counts are typically normal in patients living with hemophilia, it is generally accepted that some patients with unusually heavy bleeding or persistently long bleeds can have low hemoglobin. This anemia may be more common as a previous study revealed that patients with hemophilia had lower that average values for hemoglobin cross all ages and degrees of severity as evidenced by 31% of patients with values less than the third percentile, 46% less that then tenth percentile, and 83% less than the mean value.
Real world impact on hemogram in pediatric patients who have been switched to Emicizumab has not been reported in the past after the medication was approved by FDA for hemophilia management.
Methods: We performed a retrospective chart review of a cohort of 12 male pediatric patients with hemophilia A who were transitioned from factor VIII to emicizumab prophylaxis between May 2019 and May 2022 to determine changes in laboratory values and bleeding frequencies.
Results:
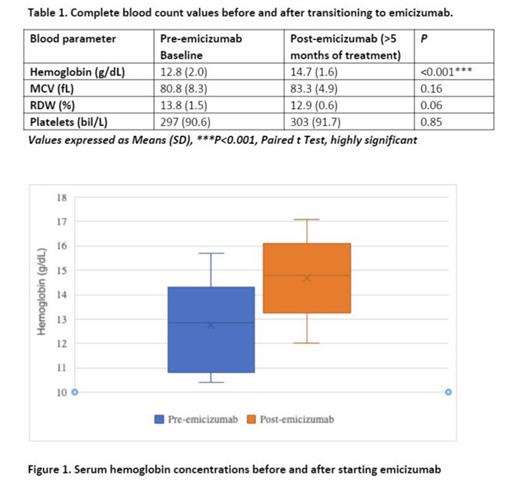
Hematologic metricies in severe hemophilia patients pre and post emicizumab switch :Complete blood counts before emicizumab transition revealed a mean hemoglobin 12.8 g/dL (range 10.4-15.7 g/dL), MCV 80.8 fL (range 67.2-99.1 fL), RDW 13.8% (range 11.8-16.6%), and platelets 297 bil/L (range 166-490 bil/L). Following emicizumab initiation, follow up blood counts demonstrated mean hemoglobin 14.8 g/dL (range 12.0-17.1 g/dL), MCV 83.3 fL (range 77.2-95.4 fL), RDW 12.9% (range 12.0-13.9%), and platelets 303 bil/L (range 199-514 bil/L). Mean hemoglobin was noted to have a significant increase after emicizumab was started (P<0.001). Both minor and breakthrough bleeding frequencies were noted to decrease in all patients.
Bleeding Frequency and Overall Satisfaction : The patient /caregiver input to assess changes in bleeding frequency was obtained, after stable treatment with emicizumab was tolerated for at least 6 months. The bleeding frequencies of our cohort of patients on FVIII prophylaxis had a wide range with minor bleed frequencies (defined as any joint, soft tissue or other hemorrhage not requiring additional FVIII administration) occurring as often as daily to once yearly. Breakthrough bleeds that required additional episodic FVIII administration occurred 2-3x/monthly to once yearly. Following transition to emicizumab, patients had a reported an overall decrease in bleeding frequencies with minor bleeds averaging from 2x/month previously to never. 3 of these patients had breakthrough bleeding episodes requiring FVIII with 2 episodes attributed to trauma.
Overall, patients' parents were asked to give a subjective verbal rating from 1 to 10 of their experience with emicizumab with 1 being worst and 10 being best. The mean verbal rating of our cohort of patients was 9.3. 11 of 12 patients remain of emicizumab prophylaxis as of May 2023 with one patient returning to FVIII prophylaxis due to pain at injection site.
Conclusion: An increase in mean hemoglobin concentration in conjunction with reported decreased bleeding frequencies may suggest emicizumab facilitates an overall reduction in prolonged bleeding episodes. At our center, hemophilia A patients transitioned from factor VIII to emicizumab prophylaxis demonstrated a statistically significant increase in hemoglobin concentrations. This data combined with a notable reported decrease in minor and breakthrough bleeding frequencies in all patients suggests an overall reduction in prolonged bleeding episodes. Patients and their families appear to be very satisfied with emicizumab prophylaxis which may promote changes in the standard of care for hemophilia A patients.
Disclosures
Jain:GBT: Honoraria; Octapharma: Honoraria; Bayer: Speakers Bureau; Takeda: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal